
Diamond is a three-dimensional network-like structure, in the diamond structure, a carbon atom having a tetravalent state, i.e. sp3 hybrid state. The diamond structure of the basic characteristics of each carbon atom with 4 neighboring atoms are connected, they are in the tetrahedral Apex directions, each carbon atom with 4 neighboring carbon atoms shared fourth on the price of the electronic form 4 covalent bonds with the surrounding atoms connected to form a tetrahedron. The diamond crystal is composed of many tetrahedral superposition. Any Out 2 adjacent to the covalent bond, each of the two single bonds attributed to the two six-membered rings at all, and not only owned a six-membered ring all as shown in the figure, the Red two-carbon single bond, it may constitute a blue and purple the two six-membered rings in.
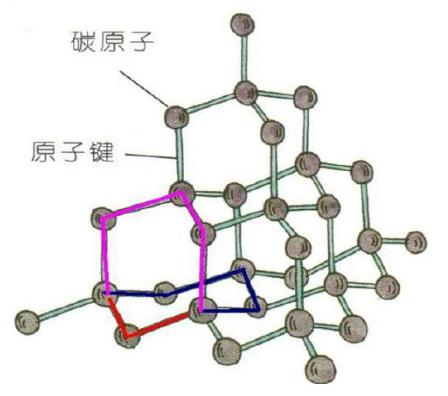
Crystal lattice, an atom with four adjacent atoms having a regular tetrahedron structure, the presence of the cubic crystal system and a hexagonal system two possibilities, natural diamond and synthetic diamond are generally cubic crystal of diamond, but there is also a hexagonal Diamond. To have both crystal structure of diamond are referred to as cubic diamond and hexagonal Diamond.
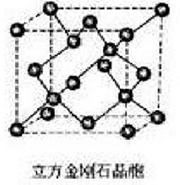
Cubic Diamond crystal structure:
Cubic Diamond is an isometric crystal xi2, at atmospheric pressure and room temperature lattice constant of 0. 356-0. 327 nm. This crystal structure can be divided into many of the same cubic unit cell, crystal cell on the surface of the atom distribution formed just a face-centered cubic structure, the unit cell has four atoms, each with one Apex Atom and the three adjacent face-centered atoms equidistant, and covalently linked, forming a tetrahedron structure.
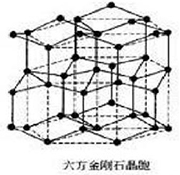
Hexagonal Diamond crystal structure:
The Hexagonal Diamond is a hexagonal crystal system, the lattice constant is a =0.252 nm and c =0.412 nm. This crystal structure can be divided into many of the same hexagonal lattice, each Atom with four adjacent atoms in a covalent bond coupling, having a regular tetrahedron structure.
Prev:Diamond performance
Next:Diamond synthesis
PRODUCTS:
Copyright © 2018 Henan Baililai Superhard Materials Co., Ltd. All rights reserved. Powered by MetInfo
TOP

 简体中文
简体中文 English
English Pусский
Pусский
.jpg)